If you have ever looked at a complex natural product and wondered, “How on earth did they build that ring system so cleanly?”, the Robinson annulation is one of the classic answers. And because students and working chemists ask it constantly, let’s tackle the big question head-on: What are the Two Starting Materials for a Robinson Annulation?
In the simplest, most practical sense, the Robinson annulation is a one-pot strategy that stitches together a new six-membered ring by combining a Michael addition with an intramolecular aldol condensation, typically ending in a cyclohexenone (an alpha, beta-unsaturated ketone).
So what do you actually need on the bench to make it work? You need two partners that “want” to do those two steps in sequence.
The short answer first: the two starting materials
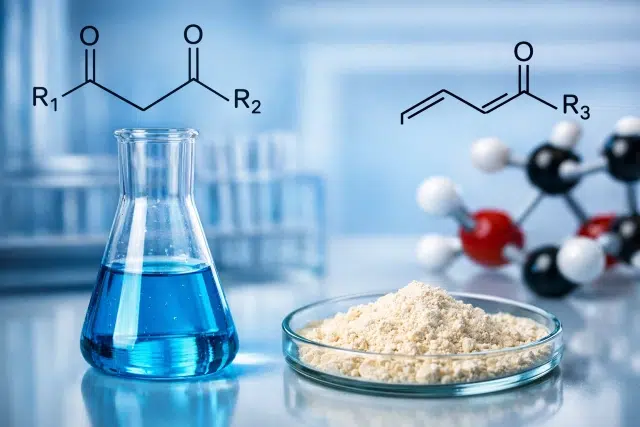
When people ask What are the Two Starting Materials for a Robinson Annulation, they are usually asking for the two carbonyl-containing partners that kick off the cascade:
- A Michael donor that can form an enolate or enamine
Most commonly: an enolizable ketone, β-diketone, or β-keto ester (something with acidic alpha-hydrogens that can generate a nucleophile). - A Michael acceptor that is electrophilic at the beta-carbon
Most commonly: an α,β-unsaturated carbonyl compound, especially an enone such as methyl vinyl ketone (MVK) or related vinyl ketones/chalcones.
Those two are the core. Everything else (base, solvent, temperature, catalysts) is there to help them meet, react in the right order, and not misbehave.
Why these two starting materials are not random
The Robinson annulation is not just “mix two things and hope.” It is designed to force a sequence:
- Step 1: Michael addition makes a 1,5-dicarbonyl-type intermediate.
- Step 2: Intramolecular aldol condensation closes the ring and then dehydration gives the enone.
That sequence only works smoothly if your two starting materials are matched:
- one partner can become a nucleophile (donor),
- the other partner is a strong conjugate acceptor (acceptor),
- and the resulting intermediate contains the geometry and functionality needed to cyclize.
This is why the “two starting materials” question matters so much: choose them well and the reaction feels almost inevitable.
What are the Two Starting Materials for a Robinson Annulation in real lab language?
Here is the same answer, but translated into how chemists talk about it in practice.
Starting material 1: the Michael donor (enolate or enamine source)
This is typically:
- a ketone (cyclohexanone is a classic),
- or a 1,3-dicarbonyl compound like a β-diketone or β-keto ester.
Why those? Because they form enolates easily under base (or enamines under amine catalysis), and those enolates do conjugate addition very reliably.
Common donor examples:
- cyclohexanone
- cyclopentanone
- acetylacetone (a β-diketone)
- ethyl acetoacetate (a β-keto ester)
OpenStax even calls out β-diketones and β-keto esters as typical enolate sources for the first step.
Starting material 2: the Michael acceptor (α,β-unsaturated carbonyl)
This is most often:
- an α,β-unsaturated ketone (enone), like methyl vinyl ketone,
- or a related conjugated carbonyl such as a chalcone-type enone.
The acceptor must be activated enough for 1,4-addition so the donor adds at the beta-carbon, not by 1,2-addition to the carbonyl.
Classic acceptor examples:
- methyl vinyl ketone (MVK)
- ethyl vinyl ketone
- substituted enones
- chalcones (common in cyclohexenone-building variants)
A practical table: donor vs acceptor at a glance
| Role in Robinson annulation | What it must do | Typical functional type | Common examples |
|---|---|---|---|
| Michael donor | Form an enolate/enamine nucleophile | Enolizable ketone, β-diketone, β-keto ester | cyclohexanone, 2-methyl-1,3-cyclohexanedione, ethyl acetoacetate |
| Michael acceptor | Accept conjugate (1,4) addition | α,β-unsaturated carbonyl (enone) | methyl vinyl ketone, substituted vinyl ketones, chalcones |
If you remember nothing else from What are the Two Starting Materials for a Robinson Annulation, remember this pairing: enolate-former + enone.
The “classic” example everyone teaches (and why it’s so famous)
A clean teaching example uses:
- cyclohexanone (donor)
- methyl vinyl ketone (acceptor)
LibreTexts describes the logic clearly: the Michael addition forms a 1,5-dicarbonyl fragment, which then undergoes intramolecular aldol condensation to yield the cyclohexenone.
But there’s an even more famous named intermediate in synthesis: the Wieland–Miescher ketone, a workhorse building block in steroid and terpenoid synthesis. It is prepared by a Robinson annulation of 2-methyl-1,3-cyclohexanedione (donor) and methyl vinyl ketone (acceptor).
That example is not famous just because it works. It’s famous because it shows what the Robinson annulation does best: build a highly useful ring system efficiently.
What actually happens between the two starting materials?
People often memorize the answer to What are the Two Starting Materials for a Robinson Annulation but don’t feel the “why.” So here’s the intuitive story.
1) The donor becomes a nucleophile
A base (or amine catalyst) pulls off an acidic alpha hydrogen, giving an enolate (or enamine).
2) The nucleophile adds to the acceptor (Michael addition)
The enolate attacks the beta-carbon of the α,β-unsaturated carbonyl to form a new carbon-carbon bond, producing a new enolate that gets protonated.
3) The new intermediate cyclizes (intramolecular aldol)
Now you have an arrangement where an enolate can attack another carbonyl within the same molecule. That closes a six-membered ring, producing a beta-hydroxy carbonyl (an aldol product).
4) Dehydration gives the final enone
Loss of water (often under heating) yields the conjugated cyclohexenone.
That’s why the starting materials have to be what they are: one must create the nucleophile, and the other must accept it in a way that sets up the internal aldol.
Variations you will actually see in problems and papers
Even though the classic pairing is “ketone + enone,” real-world Robinson annulations come in a few flavors.
Variation A: β-diketone (or β-keto ester) donors are popular for a reason
They form enolates easily, and regioselectivity can be cleaner because the most stabilized enolate is strongly favored.
This is one reason the Wieland–Miescher route uses a 1,3-dicarbonyl donor.
Variation B: chalcones and substituted enones as acceptors
In synthetic methods, chalcone-type acceptors are common when the goal is substituted cyclohexenones, and ScienceDirect notes this “condensation of chalcones with 1,3-dicarbonyl compounds” framing as a traditional route to cyclohex-2-enones.
Variation C: different activation modes (base, acid, organocatalysis)
Most textbook examples are base-promoted, but acid catalysis exists, and some modern protocols reduce side reactions by controlling the enone concentration (for example, generating the enone in situ from a precursor).
There are also organocatalytic approaches (often proline-based) used in enantioselective variants of the Robinson annulation, especially around the Wieland–Miescher system.
Actionable tips for choosing the two starting materials (the stuff that saves you time)
If you are using What are the Two Starting Materials for a Robinson Annulation as a checklist for planning, here are the practical filters chemists use.
Pick a donor that enolizes where you want it to
Ask:
- Does it have alpha-hydrogens?
- Will it form the “right” enolate (kinetic vs thermodynamic)?
- Would a 1,3-dicarbonyl donor make the site obvious and selective?
Pick an acceptor that behaves like a Michael acceptor, not a trap
Enones can polymerize or get messy, especially reactive ones like MVK. Some protocols manage this by using enone precursors to keep the steady-state concentration low.
Think ahead to the aldol closure
A simple rule: after the Michael step, your intermediate must be able to fold into a six-membered ring and present an electrophilic carbonyl at the right distance. LibreTexts emphasizes that it’s the 1,5-dicarbonyl motif that makes intramolecular aldol condensation “ready to go.”
Common questions people ask (FAQ)
What are the Two Starting Materials for a Robinson Annulation in one sentence?
What are the Two Starting Materials for a Robinson Annulation: an enolate-forming carbonyl compound (often a ketone or 1,3-dicarbonyl) plus an α,β-unsaturated carbonyl compound (often an enone such as methyl vinyl ketone).
Does it have to be a ketone and methyl vinyl ketone?
No. MVK is a classic acceptor because it is very effective, but many substituted enones work, and donors can be ketones, β-diketones, or β-keto esters depending on what you want to build.
Why does the product usually end as a cyclohexenone?
Because the sequence is designed to form a six-membered ring via intramolecular aldol condensation, then dehydrate to the conjugated enone, which is thermodynamically favored.
Can the Robinson annulation be asymmetric?
Yes. Enantioselective approaches exist (including organocatalytic variants) and are often demonstrated on the Wieland–Miescher ketone platform.
Where is Robinson annulation used in real synthesis?
It shows up in natural product synthesis and complex ring construction, including classic steroid-related intermediates like the Wieland–Miescher ketone and examples in estrone synthesis discussions.
A quick mini case study: building a steroid-like ring junction
If you want a feel for why this reaction is still taught (and still used), look at how frequently the Robinson annulation is used as a ring-building step toward steroid-like frameworks. Educational texts point out its role in routes that produce cyclohexenone rings, and discussions of estrone synthesis include a Robinson annulation step as a representative example.
And the Wieland–Miescher ketone example is especially telling: a single annulation step constructs a highly versatile bicyclic core that has been used widely in total synthesis.
Putting it all together (a simple mental checklist)
When you see a Robinson annulation problem, run this checklist:
- Identify the Michael donor: where is the most reasonable enolate (or enamine) coming from?
- Identify the Michael acceptor: where is the α,β-unsaturated carbonyl that can take 1,4-addition?
- Confirm the Michael product creates a 1,5-dicarbonyl-type arrangement that can cyclize.
- Expect the final product to be a substituted cyclohexenone after aldol plus dehydration.
Conclusion
So, What are the Two Starting Materials for a Robinson Annulation? At its core, it is the pairing of a Michael donor that can form an enolate (or enamine) with a Michael acceptor that is an α,β-unsaturated carbonyl compound. That donor-acceptor match is what makes the domino sequence work: Michael addition first, then intramolecular aldol condensation, then dehydration to a cyclohexenone.
Once you start spotting those two partners in structures, the reaction stops feeling like a “named reaction to memorize” and starts feeling like a practical ring-building tool you can plan with. If you want a fast refresher on the named-reaction background, this reaction mechanism overview is a handy reference.